COMPANY
Quality
Our goal is to stay at the forefront to offer you new solutions every day

Guaranteed quality since 1983
The production processes, evaluated and validated by the international certifier IMQ, meet the highest quality standards, which accredits Microdent with the corresponding CE mark for medical devices as well as the international certificates ISO 13485, ISO 9001 and the recommendations of ISO 10993 in terms of cytotoxicity, implant surface residue, intra-cutaneous reactivity and systemic toxicity.
Certified Quality
Microdent's main objective is to offer its customers products with the maximum guarantee of efficacy and safety. To this end, all products are subject to the most rigorous quality controls, from design to direct delivery to the professional. A large number of studies endorse the quality of our products: our quality system complies with ISO 13485 and the CE mark of compliance with the European directive on medical devices 93/42/EEC granted by the IMQ Notifying Body No. 0051. It also complies with the strict regulations of the Food and Drug Administration, obtaining all FDA certificates.
Quality politics

Cytotoxicity test
Biocompatibility and biological evaluation of Microdent dental implants.
See more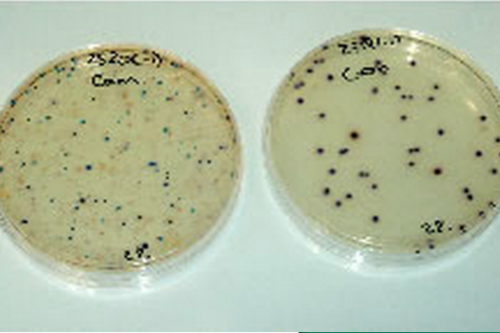
Microbiological quality assessment
The microbiological evaluation after cleaning and packaging allows to demonstrate the absence of bacteria and fungi.
See more
Fatigue tests
Fatigue studies of Microdent dental implants to determine the load limit.
See more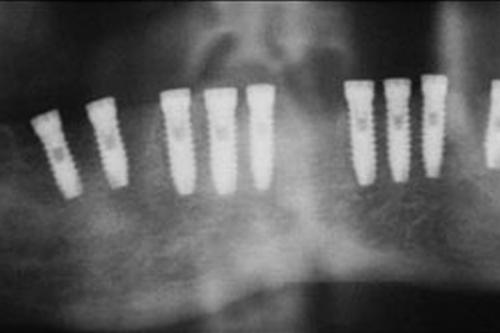
Mouth survival
Clinical follow-ups of Microdent implants have been carried out, verifying a survival rate of 97.9% of clinical cases.
See more
Quality Certificates
Products with the maximum guarantee of efficacy and safety
All our products are subjected to the most rigorous quality controls from their design to their delivery to the professional, obtaining, among others, the CE mark of compliance with the European directive on medical devices, the ISO 13485 and the certification of the Food and Drug Administration (FDA) as medical devices